Medical Corner: COVID-19 Pharmacotherapy Update
.The rapid spread of highly infective Covid-19 (SARS-CoV-2) and lack of specific pharmacotherapy until today led to unique situation for clinicians and patients - drug regimens with uncertain value while being tested in clinical trials.
The WHO SOLIDARITY Trial recently reported the interim results of major drug therapy that have generated substantial interest: 1.Remdisivir 2.Hydroxychloroquine (HCQ) 3.Lopinavir 4. Interferon Beta-1a. Among the 405 hospitals in 30 countries (from High-income countries eg. USA, Canada, France, Switzerland to Low-Middle Income (LMI) eg. Peru, Philippines) representing all six WHO regions, the 11,330 adults were randomised to receive either one of these regimens or to received hospital specific standard of care.There are no trial drugs regimen reported clinical meaningful benefit and no reduction in 28-day mortality compared to standard care: Remdisivir 0.95 (95% CI, 0.81-1.11), HCQ 1.19 (95% CI, 0.89 to 1.59), Lopinavir 1.00 (95% CI, 0.79 -1.25) and Interferon beta-1a 1.16 (95% CI, 0.96-1.39). However, the adaptive trial showed that Remdisivir reduced the number of hospital days (10 days vs 15 days with Placebo) and time to hospital discharge. This reduction in time to recovery and hospital discharge among survivors is important to overwhelmed and stressed healthcare systems. The SOLIDARITY trials send compelling message that these pharmacotherapy currently used should no longer be considered viable drug treatment options for SARS-CoV-2.
A network meta-analysis combining all available evidence published to date for Covid-19 treatment suggested that Glucocorticoids (Gl) probably reduce the need for mechanical ventilation, duration of hospitalisation and risk of death which is primarily powered based on RECOVERY trial. Remdisivir (Re) may reduced both time to symptom resolution and duration of ventilator support but the impact on death remains uncertain. There are no reported data in human clinical trials that ARBs (Angiotensin-receptor blockers) may worsen patient’s susceptibility to SARS-CoV-2.

Drug treatments for Covid-19: living systematic review and network meta analysis. BMJ 30 July 2020.; 370:m2980.
Race for Covid-19 Vaccine
Vaccine mimic the virus or part of the virus by stimulating the immune system to develop antibodies. They must follow higher safety standards than other drugs because they are given to millions of healthy population. The development of safe and effective vaccine by completing all stages in the vaccine development cycle is a daunting task in situation wherein COVID pandemic swiftly ravaged the globe in the last 6 months.
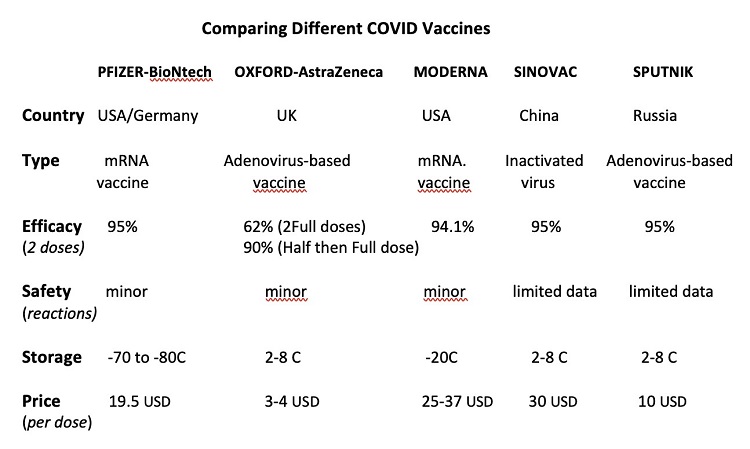
Among more than 100 candidate vaccines being developed and tracked by WHO the leading Covid vaccines are 1.Pfizer with German partner BioNtech 2.Moderna 3.Oxford-Astra Zeneca 4.Sputnik and 5.Sinovac vaccines which all claimed good efficacy in preventing acute Covid-19 symptoms based on media outlets. Currently it is only Oxford-Astra Zeneca AZD1222 and Pfizer BNT162b2 mRNA vaccines had undergone peer-review in leading scientific journals. The result published from Lancet journal of phase 3 clinical trials from Oxford-Astra Zeneca reported that this viral vector vaccine from modified adenovirus is safe with an overall efficacy of 70%. And this may induced strong antibody and T-cell response up to 56 days. Enrolled population who received the “first low dose” followed by “full dose” raised its efficacy to 90%.
Recently, the University of Queensland and the Coalition for Epidemic Preparedness (CEPI) in partnership with Australian Biotech company CSL had successfully developed a molecular clamp technology vaccine which is found to be safer and induced greater T-cell immune response compared to other vaccines. Unfortunately, Australia’s hope of a locally developed Covid-19 vaccine have been dashed and the vaccine trial was terminated due to “false positive” result to HIV tests. The vaccine’s signature “molecular clamp” technology was formulated with parts of an HIV protein. After vaccine injection the body will produced antibody which can be picked up in HIV tests conveying false result. This could lead to people think they had HIV but in fact they did not have.
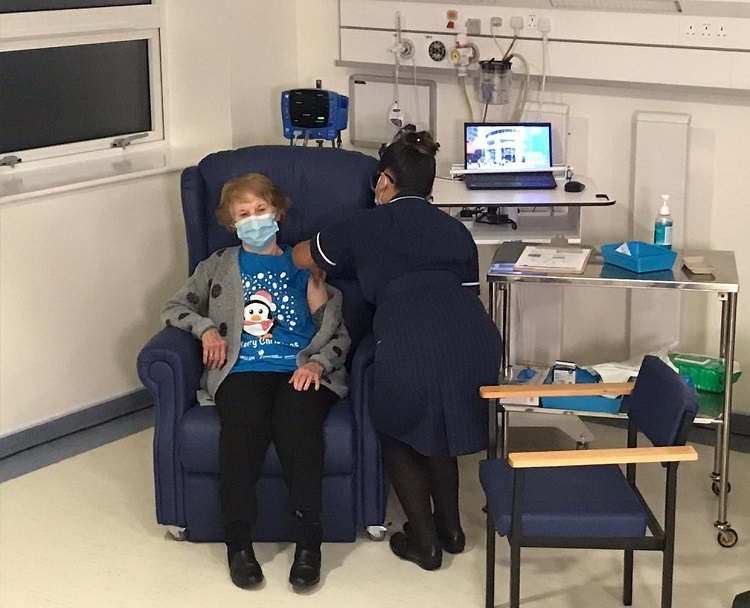
Meet Maggie, 91 yo Irish lady from UK is the first person in the world to receive COVID vaccine outside clinical trials.
The vaccine had two ways of preventing us from getting sick with COVID either it might stop you from getting infected or you may still get infection but avoid developing severe COVID disease.
Until today there are many issues remained unanswered in terms of clinical endpoint among the available vaccines being marketed: _1.How long is the immunoprotection? 2.Will you need a vaccine if you have been previously infected with COVID disease? 3.Can a person spread the virus after vaccination? 4.Is there any long-term side effects? 5.What happens to other “5 percent” who fail to benefit from a vaccine if the vaccine has efficacy of 95%?6.Are we expecting a mixed vaccine injection from different companies?
Another biggest challenge in vaccine logistics is the ability to produce them at sufficient scale to jab and cover the 7 billion population of our planet. Vaccine transport in particular the Pfizer mRNA vaccine required -70C to -80C (almost equivalent to winter season in Antarctica) to preserve its efficacy and quite problematic to carriage in LMI countries particularly among rural areas, disadvantaged community and ethnicity minority groups. The Covid-19 vaccination is one of the greatest accomplishment and complex vaccination programme in human pandemic history but it must not broaden existing health inequalities and human inequity.
Dr Ramon Joel Seastres MD. FPCP, FPCC, EDIC (Europe)
Adult Cardiology - Intensive Care Medicine
Director of Medical Program, FAHPI
Email: fahpinc@gmail.com
Canberra, Australia
